- Have any questions?
- 0809 992 8864
- info@afrabchem.com
Products

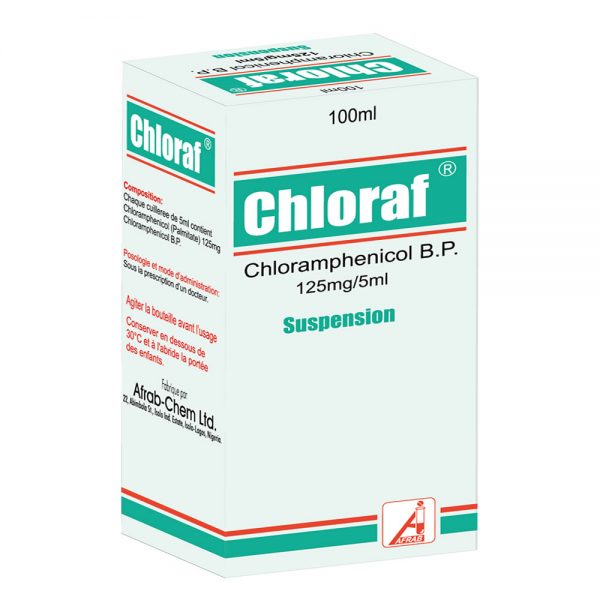
Chloraf Suspension
March 6, 2019
Nospamin Tablet
March 6, 2019Chloraf Drops
Ear Drop (NRN: 04-0325): Chloramphenicol BP 5%.
Therapeutic Class
Antibacterial, Others
Dosage Form, Composition & NAFDAC Registration Number (NRN)
Oral Suspension (NRN: 04-0543): Each 5ml (teaspoonful) contains Chloramphenicol palmitate equivalent to 125 mg Chloramphenicol BP.
Pack size: Bottle of 60ml and 100ml Suspension.
Ear Drop (NRN: 04-0325): Chloramphenicol BP 5%.
Pharmacology
Mode of action:
The mode of action is through interference or inhibition of protein synthesis in intact cells and in cell-free systems. In vitro, Chloramphenicol exerts mainly a bacteriostatic effect on a wide range of gram negative and gram-positive bacteria and is active in vitro against rickettsias, the lymphogranuloma psittacosis group and Vibrio cholerae. It is particularly active against Salmonella typhi and Hemophilus influenzae.
Potent and potentially toxic broad-spectrum antibiotic which should be reserved for life threatening infection particularly those caused by Haemophilus influenzae and Salmonella typhi.
Indications
Typhoid and paratyphoid fevers caused by S.typhi and S.paratyphi and other Salmonella infections; meningitis caused by Haemophilus influenza, Rickettsial infections and epidemic typhus, chronic infection of urinary tract caused by Proteus vulgaris which does not respond to treatment with other antibiotics.
Contra-indications
Hypersensitivity to Chloramphenicol, infections which can be treated with less toxic antibiotics, cerebral fluid infections, bone marrow disease such as leucopenia, thrombocytopenia, pregnancy and breast feeding. Avoid use in newly born babies.
Precautions/Warnings
Avoid long term treatment (not more than 10 days) and in hepatic renal impairment, regular blood cell count should be performed.
Advice to Patients:
Do not initiate or discontinue this medication without medical consultation or prescription. Do not use any medicine after “Expiry date” on the pack.
Interactions
Avoid concomitant use with anticoagulants, anti-diabetic agents, anti-epileptics; discontinue the treatment if serum iron level is high. Systemic half-life is enhanced by drugs that inhibit liver oxidase enzymes such as rifampicin, paracetamol, phenobarbitone and phenytoin. This is important in cases of poor liver dysfunction, mild toxicity to chloramphenicol and low blood count.
Adverse Effects
Agranulocytosis, aplastic anaemia, thrombocytopenic purpura and bone marrow suppression may occur in long term therapy.
Other side effects include renal toxicity, optic neuritis, jaundice, dryness of the mouth, nausea, vomiting, diarrhoea, skin rashes; increased growth of Candida albicans or other fungi on the mucous membrane may occur causing stomatitis, sore tongue and rectal or vaginal irritations.
Haemolytic anaemia in some patients with a genetic deficiency of glucose-6-phosphate dehydrogenase ashen colour of the skin, irregular respiration, perspiration, progressive pallid cyanosis, circulatory collapse and eventually death.
Prolonged administration may induce bleeding, either bone marrow depression or by reduction of intestinal flora which leads to inhibition of vitamin K synthesis hence high prothrombin time.
Dosage & Administration
25-50 mg/kg body weight daily in divided doses every 6 hours. Maximum daily dose should not exceed 2g.
Plasma concentration monitoring required in neonates and preferably also in those less than 4 years of age; recommended plasma concentration 15-25 mg/litre.
Storage/Handling Recommendations
Store in a cool dry place between 15 – 25ºC.
Keep away from the reach of children.

Reviews
There are no reviews yet.