- Have any questions?
- 0809 992 8864
- info@afrabchem.com
Products

Banedif Powder
March 5, 2019
Chemotrim Suspension
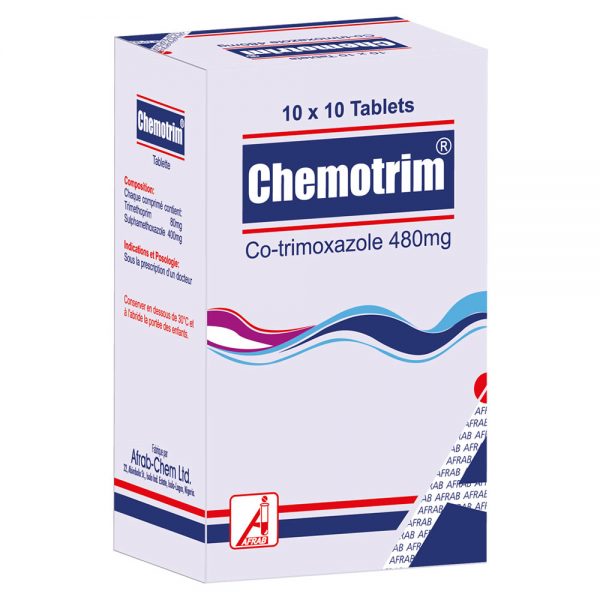
March 6, 2019Chemotrim Tablet
Tablet (NRN: 04-0219): Trimethoprim BP 80 mg and Sulphamethoxazole BP 400 mg.
Pack size: Hospital pack of 1000’s; 10 x 10’s
Therapeutic Class
Antibacterial, Sulfonamides and trimethoprim
Dosage Form, Composition & NAFDAC Registration Number (NRN)
Tablet (NRN: 04-0219): Trimethoprim BP 80 mg and Sulphamethoxazole BP 400 mg.
Pack size: Hospital pack of 1000’s; 10 x 10’s
Suspension (NRN: 04-0216): Each 5 mL contains Trimethoprim BP 40 mg and Sulphamethoxazole BP 200 mg.
Pack size: Bottles of 60 mL; 100 mL.
Pharmacology
Sulfamethoxazole inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid (PABA). Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the required enzyme, dihydrofolate reductase. Thus, Sulfamethoxazole and Trimethoprim block two consecutive steps in the biosynthesis of nucleic acids and proteins essential to many bacteria.
In vitro studies have shown that bacterial resistance develops more slowly with both Sulfamethoxazole and Trimethoprim in combination than with either Sulfamethoxazole or Trimethoprim. Sulfamethoxazole and Trimethoprim have been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections alone
Indications
Chemotrim is a broad spectrum chemotherapeutic agent widely used for the treatment of urinary tract infection (acute and chronic); respiratory tract infections (including Pneumocystis carinii); genital tract infection such as gonorrhoea; gastro-intestinal tract infection; skin and soft tissues infections.
Sensitivity testing of the culture media should be free of thymidine, a special antagonist of Trimethoprim.
Contra-indications
History of hypersensitivity to sulphonamides or trimethoprim.
Severe hepatic parenchymal damage.
Serious haematological disorders.
Neonates or premature babies.
Precautions/Warnings
Folate supplement should be considered when giving Chemotrim to suspected folate deficient patients and to the elderly or in prolonged high doses.
Chemotrim should be used cautiously and in reduced dosage in patients with impaired renal function because of the risk of crystalluria. Adequate fluid intake should be maintained and the administration of alkalis may be necessary if very large doses are used.
In pregnancy and lactation: The safety of Chemotrim in human pregnancy has not been established. Sulphonamide containing products should not be administered in the late pregnancy because of the risk of kernicterus.
Interactions
Chemotrim has been shown to potentiate the anticoagulant activity of warfarin. Careful control of anticoagulant therapy during treatment with Chemotrim is advisable.
Chemotrim prolongs the half life of phenytoin and may affect thyroid tests.
Adverse Effects
Most common side effects include mild nausea with or without vomiting and skin rashes; various haematological changes have been reported, the majority being mild and reversible when treatment was stopped.
Dosage & Administration
Preferably taken on a full stomach with some food or drink to minimize the possibility of GI disturbances.
Paediatric Suspension:
| Age | Dosage |
| Infants (6 weeks – 5 months) | 2.5-5 mL (½-1 teaspoonful) every 12 hours |
| 6 months – 5 years | 5 mL (1 teaspoonful) every 12 hours |
| 6-12 years | 10 mL ( 2 teaspoonfuls) every 12 hours |
Tablets:
| Age | Dosage | |
| Children over 12 years and Adults | In mild infections | 2 tablets every 12 hours |
| In severe infections | 2 tablets every 8 hours | |
Or as directed by physician.Note: At least, 5 days treatment is recommended. In severe infections for all age groups, dosage may be increased by 50%. Treatment should continue until patient is symptoms free.
Low doses are given for long term treatment and in patients with renal impairment. Chemotrim is generally not recommended when creatinine clearance is less than 5 ml per minute unless facilities for haemodialysis are available.
Gonorrhoea: 4 tablets every two hours or 5 tablets followed by a further dose of 5 tablets eight hours later.
Pneumocystis carinii Pneumonia: 120 mg per kg body weight daily, given in 2 or 4 divided doses for 14 days. Blood concentration of sulphamethoxazle should be monitored.
Overdosage:
Nausea, vomiting, dizziness and confusion are ikely symptoms of overdose. Gastric lavage may be useful though absorption from the GIT is very rapid and complete in approximately two hours. Acidification of the urine may enhance the rate of elimination of Trimethoprim. Calcium folinate (3-6 mg/day) will counteract effect of trimethoprim on the bone marrow.
Storage/Handling Recommendations
Store all medicines in a cool dry place.

Reviews
There are no reviews yet.